FreeStyle Libre 2 have been upgraded to FreeStyle libre 2 Plus. There is a price increase which directly relates to the additional day of wear time which is 15 days.
 Menu
Menu
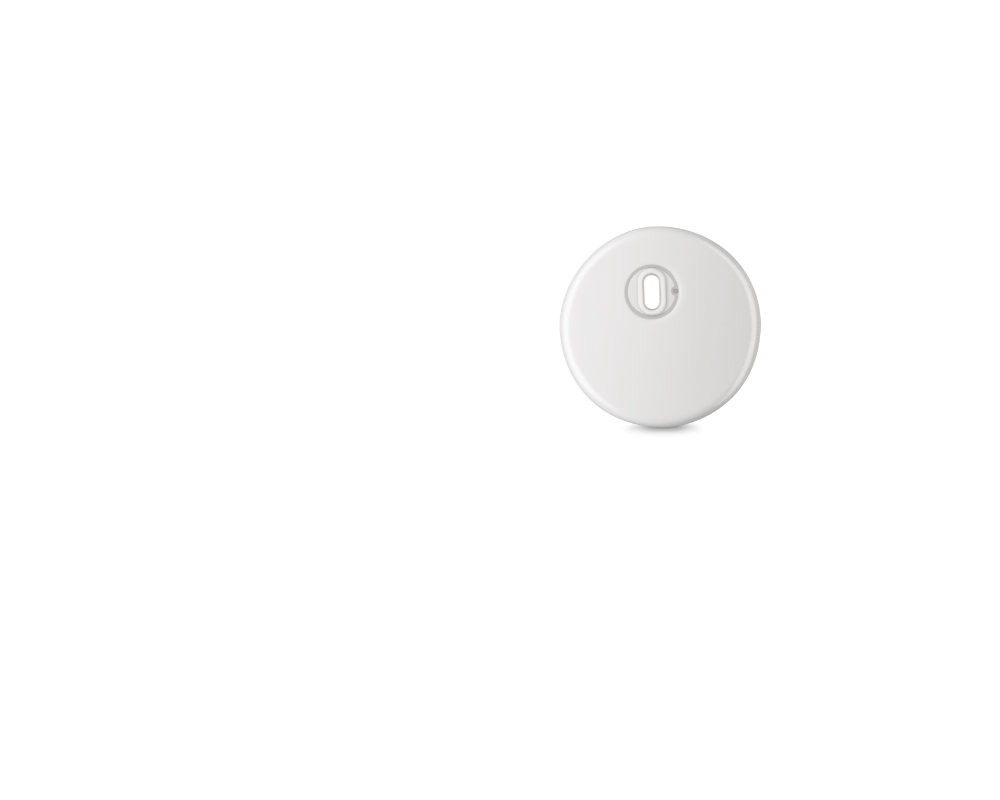
Pair the accurate1 , minute-to-minute glucose readings from your FreeStyle Libre 3 Plus sensor with your Automated Insulin Delivery (AID) system
The FreeStyle Libre 3 Plus sensor is the latest innovation to the FreeStyle Libre system. The FreeStyle Libre 3 Plus sensor is currently only available in New Zealand for people who are accessing funded Automated Insulin Delivery (AID) systems through Pharmac.
The FreeStyle Libre 3 Plus sensor is not currently available as a standalone CGM device in New Zealand. The FreeStyle Libre 3 Reader or FreeStyle Libre 3 app is not currently available for use or purchase in New Zealand.

The FreeStyle Libre 3 plus sensor is the world's smallest CGM sensor*, with an extended wear time of up to 15 days, and the only CGM sensor in New Zealand that takes a glucose reading every single minute.*
Clinically proven to lower your HbA1c.2
Reduced hypoglycaemia - spend less time in the low glucose range, both during the day and night. 3-6
Clinically proven to increase time spent in your target glucose range. 6-8 More time in range is linked with a lower HbAlc.9
The system runs a smart quality check, every single minute, to ensure each reading consistently reliable.
|
|
 |
 |
 |
|
The FreeStyle Libre 2 sensor is funded for eligible users looking for a standalone CGM, and not using an insulin pump /automated insulin delivery (AID) system. |
The FreeStyle Libre 3 Plus sensor is funded for eligible users with authorised insulin pump/ automated insulin delivery (AID) system. |
|
5mm x 35mm |
2.9mm x 21mm |
| Up to 14 days wear time | Up to 15 days wear time |
| Connects with FreeStyle LibreLinkØ and LibreLinkUpΩ | No apps currently available in NZ |
| 6 meters unobstructed Bluetooth range | 10 meters unobstructed Bluetooth range |
|
Worn on the back of the upper arm |
Worn on the back of the upper arm |
|
No calibration required |
No calibration required |
| Automatic glucose readings every single minute | Automatic glucose readings every single minute |
|
Free from IBOA and MBPA (common alergens)10 |
Free from IBOA and MBPA (common alergens)10 |
| For people not using AID systems |
Authorised to work with the mylife Ypso Pump with CamAPS FX, and the Tandem t:slim x2 pump^for automated insulin delivery |
|
Available for people with insulin requiring diabetes, aged 4+ |
Available for people with diabetes, aged 2+ |
In New Zealand, the FreeStyle Libre 3 Plus sensor is authorised to work with select insulin pump/AID systems which include the mylife YpsoPump from 1st October 2024 and the Tandem t:slim X2 insulin pump by July 2025.
To check if your phone is compatible with the FreeStyle Libre 3 Plus sensor when used with the mylife YpsoPump,please refer to the FreeStyle Libre 3 system phone compatibility guide here to see if your phone/operating system is listed.
Your insulin pump may also have specific phone compatibility requirements, so please consult your healthcare professional or the insulin pump manufacturer.
If you have any questions, please contact our friendly customer care team at
0800 106 100 (option 2).

The FreeStyle Libre 3 Plus sensor is not available as a standalone CGM device in New Zealand and is not currently compatible with devices and tools in the FreeStyle Libre system, including the FreeStyle Libre reader, FreeStyle Libre 2 reader, and the FreeStyle LibreLink app. This also means that sensor glucose readings cannot be uploaded to LibreView, and cannot be shared with LibreLinkUp connections.
The FreeStyle Libre 3 Plus sensor is compatible with select insulin pump/Automated Insulin Delivery (AID) systems and will transmit glucose data directly to the insulin pump manufacturer’s device. Real-time FreeStyle Libre 3 Plus sensor glucose readings are displayed on the insulin pump manufacturer's reading device, such as a smartphone app. The insulin pump manufacturer's device is the only compatible method for users to view FreeStyle Libre 3 Plus sensor glucose readings.
The FreeStyle Libre 3 Plus sensor measures 21 x 2.9 mm. This is roughly equivalent to two stacked 10c coins.
The FreeStyle Libre 3 Plus sensor is indicated for use to be worn on the back of the upper arm.
The FreeStyle Libre 3 Plus sensor is water-resistant and can be worn while bathing, showering, or swimming.* Please note that Bluetooth performance may be impacted if using the system while underwater.
*Sensor is water-resistant in up to 1 metre (3 feet) of water. Do not immerse longer than 30 minutes.
The FreeStyle Libre 3 Plus sensor has the same adhesive as the FreeStyle Libre 2 sensor.
Currently you are unable to purchase FreeStyle Libre 3 Plus sensors from the Mediray website.
1. U.S. Food and Drug Administration (FDA). 510(k) K222447. 2023. https://www.accessdata.fda.gov/cdrh_docs/reviews/K222447.pdf. Accessed 28 Aug 2024.
2. Evans M. et al. Diabetes Ther. 2022; 13(6): 1175-85.
3. Leelarathna L. et al. N Engl J Med. 2022; 387:1477-1487.
4. Franceschi R. et al. Front. Endocrinol. 2022; 13:907517.
5. Haak T.et al. Diabetes Ther. 2017; 8(1): 55-73.
6. Bolinder J. et al. Lancet 2016;388(10057): 2254-2263.
7. Campbell FM. et al. Pediatr Diabetes. 2018; 19:1294–1301.
8. Ogawa W. et al . J Diabetes Invest. 2021; 12(1): 82–90.
9. Beck RW. et al. J Diabetes Sci Technol. 2019; 13(4): 614–26.
10.Seibold. A. J Diabetes Sci Technol. 2021;15(3):713-714.
*Compared to other on-market CGMs. As of Aug 2024.
Ω The LibreLinkUp app is only compatible with certain mobile device and operating systems. Please check www.librelinkup.com for more information about device compatibility before using the app. Use of LibreLinkUp and FreeStyle LibreLink requires registration with LibreView. The LibreLinkUp mobile app is not intended to be a primary glucose monitor: home users must consult their primary device(s) and consult a healthcare professional before making any medical interpretation and therapy adjustments from the information provided by the app, have similar but not identical features.
The FreeStyle Libre 2 CGM System is indicated for measuring interstitial fluid glucose levels in people (aged 4 and older) with insulin-dependent diabetes. Always read the instructions for use. The system must not be used with automated insulin dosing (AID) systems, including closed-loop and insulin suspend systems. The sensor must be removed prior to Magnetic Resonance Imaging (MRI). The FreeStyle Libre 3 Plus CGM System is indicated for measuring interstitial fluid glucose levels in people (aged 2+) with diabetes mellitus. Always read the instructions for use. The sensor must be removed prior to Magnetic Resonance Imaging (MRI).
The sensor housing, FreeStyle, Libre, and related brand marks are marks of Abbott. Information contained herein is for distribution outside of the USA only. Mediray New Zealand, 53-55 Paul Matthews Road, Albany, Auckland 0632. NZBN 9429041039915. ABN 95 000 180 389. ADC-99968 V1.0